INTRODUCTION (Added December 28, 2020)
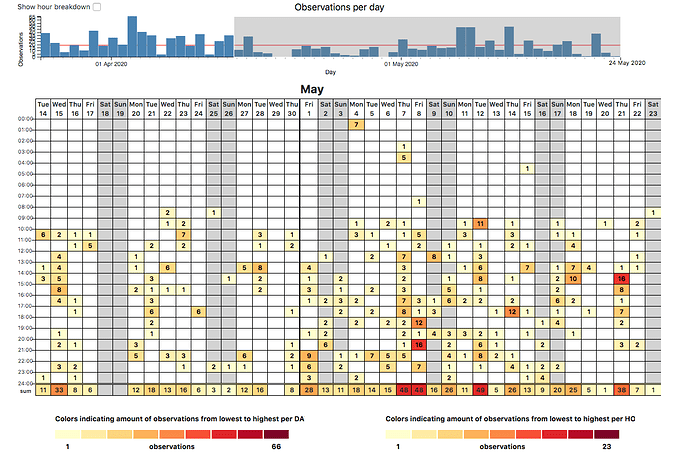
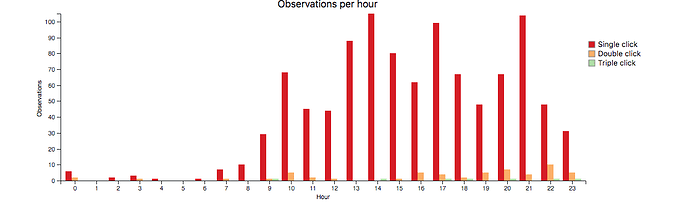
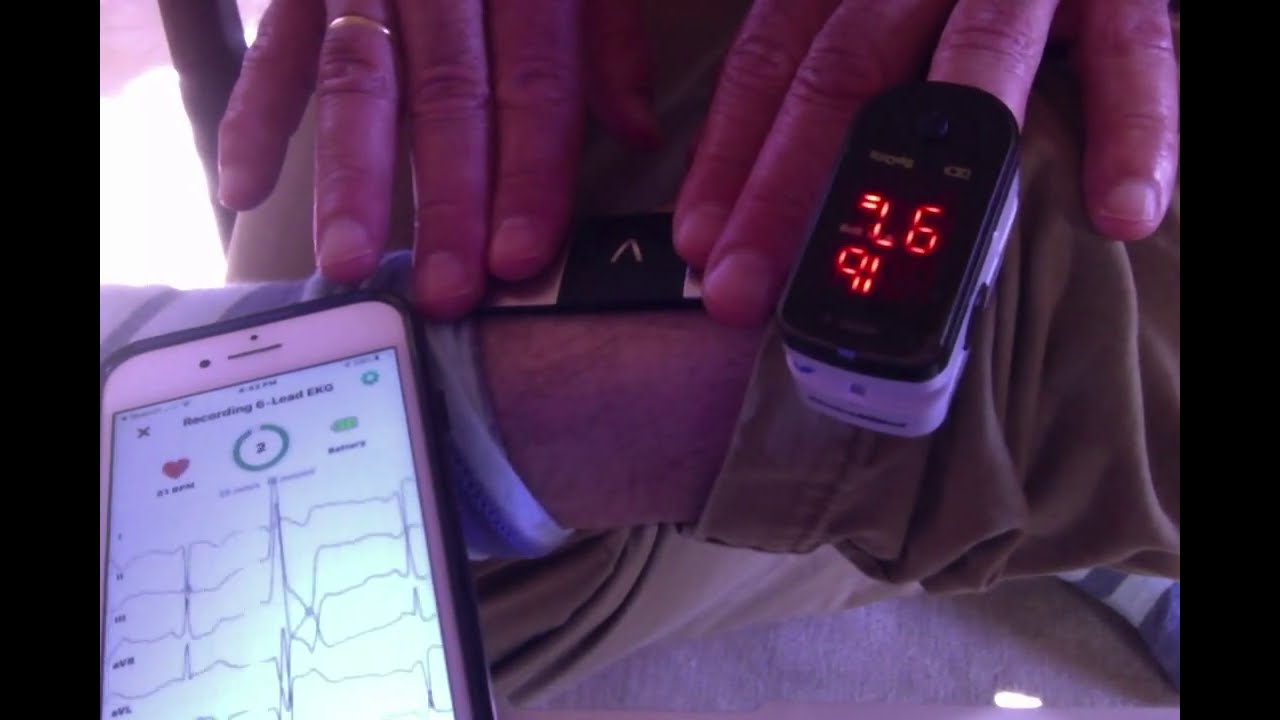
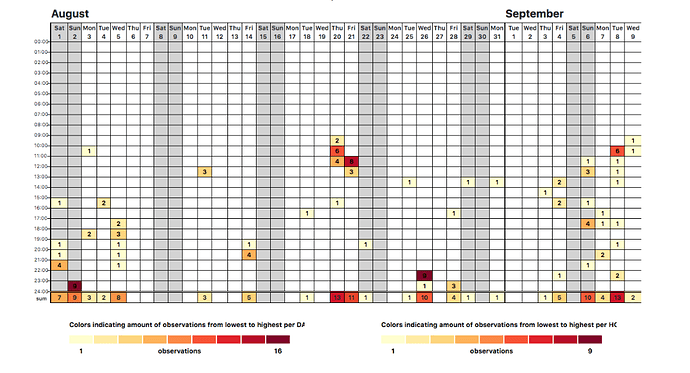
This is a project log about tracking my arrhythmias. The notes begin with some journaling reports and quickly move into tracking arrhythmia events using the 1-Button Tracker. In September I begin tracking with a biomedical device (the Zio patch) and run a correlation test between the Zio and the 1-Button method. In November I began taking metoprolol, a beta blocker, and tracking doses while also continuing to track events with the 1-Button. As of today I haven’t yet run an analysis of the medication vs events, though it is obvious the medication is helping.
Original 1st post continues below:
I just created an account on EventLoggers, a journaling and data integration tool created by @dreeds, who is active on this forum. It seemed like it could be useful to me in a project I’m doing to investigate the triggers of my arrhythmias. Through some self-tracking and sharing my data in a video conference with a cardiologist, I’ve learned that these are most probably all premature ventricular contractions (PVCs). It’s a common condition, and many people find they are triggered by alcohol, coffee, stress, sleep problems. I’m not sure enough about what’s going on to do a very rigorous experiment. My tolerance for controlling my daily life through very careful timing of events and lifestyle changes is not very high, so before I try to do something difficult I want to know more about what’s going on. So I’m going to keep a journal on EventLoggers for two weeks and then assess.
I normally drink about 5 drinks a week, rarely more than 1 to 1.5 ounces of 80 proof liquor per drink. I’ve been leaning against this and haven’t had a drink since Sunday night, and also haven’t had a severe arrhythmia period since Tuesday. So – a more or less natural experiment is already under way. Eventually I’ll have another drink, certainly by this Sunday when some in-laws come over for a (well distanced) visit outside on our lawn. I’ve also limited myself to a single cup of coffee in the morning.
What is responsible for my improvement? Less coffee or no alcohol? Or both? Or something else all together? Over time, I hope to find out more.